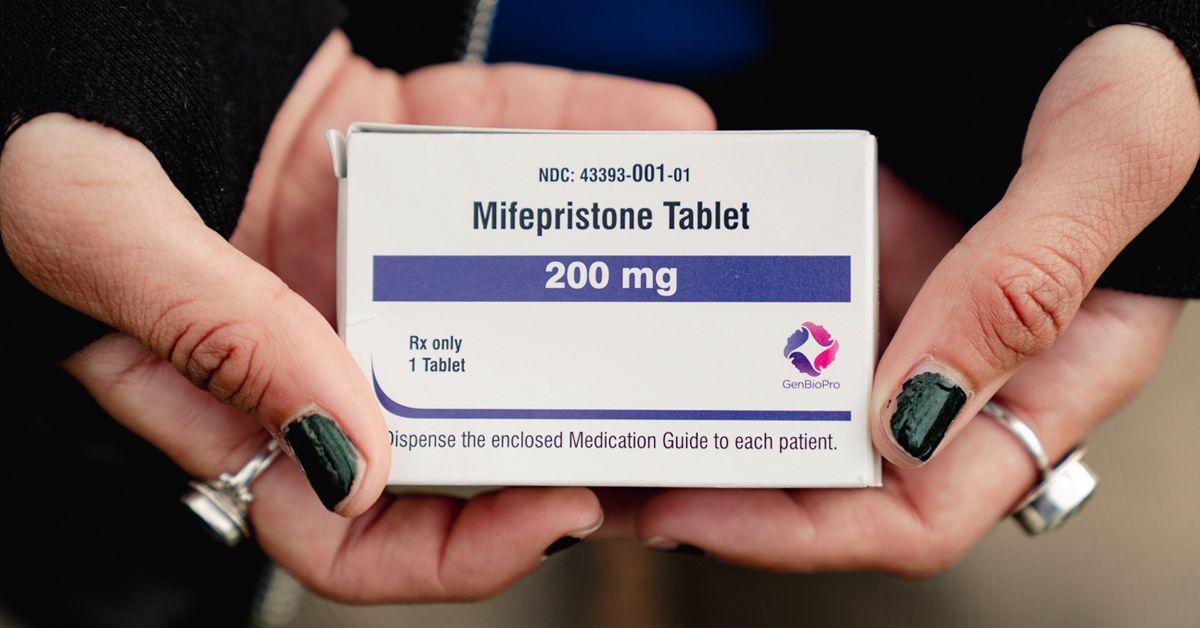
The Food and Drug Administration (FDA) has approved a generic version of mifepristone, the medication used with misoprostol for medication abortion up to 70 days’ gestation. Evita Solutions, LLC received approval under Abbreviated New Drug Application (ANDA) 216616 for 200 mg tablets that the FDA found bioequivalent and therapeutically equivalent to the brand-name product Mifeprex, made by Danco Laboratories.
This approval is effective immediately and is expected to increase affordability and availability of medication abortion by providing a lower-cost alternative to the branded drug. Evita Solutions anticipates its product will be available by January 2026. Another manufacturer, GenBioPro, has already supplied a generic mifepristone product for several years.
Safety and regulation
Mifepristone — whether branded or generic — is regulated under the same safety standards. It is distributed within a Risk Evaluation and Mitigation Strategy (REMS) program designed to ensure benefits outweigh risks. The REMS for mifepristone is a shared system across manufacturers and requires provider certification, patient agreements, and controlled distribution before products enter interstate commerce. The FDA’s approval letter to Evita reiterates that the generic will join this shared safety framework.
Experts note that the controversy around mifepristone is mainly political and ideological rather than scientific. Decades of research support mifepristone’s safety and effectiveness when used as directed. Physicians follow established guidelines for prescribing and follow-up care to ensure appropriate counseling and monitoring.
Access, cost, and insurance
A generic option generally reduces cost, which can improve access for people who face barriers such as geography, finances, or stigma. However, insurance coverage for the new product will vary. Health plans may add the generic to formularies quickly or wait until a plan-year update; they are not required to include it. State laws also play a large role: some states restrict abortion and prohibit coverage for abortion-related care, while others require coverage. Because mifepristone is also used to manage miscarriages, some plans in restrictive states may cover it for that indication even if they block coverage for abortion.
To determine coverage, individuals should contact their health plan to learn whether mifepristone is covered, whether coverage is limited to miscarriage management or extends to all approved uses, and what out-of-pocket costs to expect.
Impact on reproductive care
The FDA approval of an additional generic mifepristone product represents a step toward broader access to evidence-based reproductive healthcare. Experts emphasize that reducing cost, maintaining rigorous safety protocols, and addressing stigma around abortion and sexual and reproductive health are important to improving outcomes and ensuring people can make informed healthcare decisions.
People seeking medication abortion or treatment for miscarriage should consult a licensed healthcare provider to discuss options, eligibility, and safe use under REMS requirements, and should check their insurance plan for coverage details.